Next Media animation scripted by Katie Golde/MEDILL
Music: “Mellowtron” Kevin MacLeod (incompetech.com)
Licensed under Creative Commons: By Attribution 3.0
http://creativecommons.org/licenses/by/3.0/
Alafair’s hydrogel film is intended to reduce the development of soft tissue attachment post surgery on a human tendon. That can help maintain mobility.
BY KATIE GOLDE
MAR 13, 2014
Imagine cutting your finger, severing your tendon and undergoing surgery for repair.
After the procedure, you realize you no longer have a full range of motion with your finger – a fairly common surgical complication that results from the addition of fibrous tissue growths around your injured tendon. Suddenly, simple acts like minor household repairs, braiding your hair or grabbing the bar for a chin up aren’t possible due to your limited mobility.
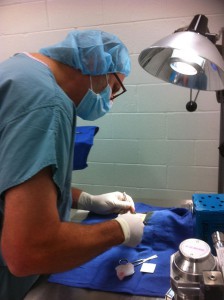
Alafair Biosciences
Dr. Dan Peterson, Alafair’s CEO and practicing neurosurgeon, performs surgical hydrogel testing in an animal model.
In Austin, a team of biomedical researchers and business experts at Alafair Biosciences are developing a product that will reduce the chances of this side effect.
While the product remains unnamed, Alafair’s medical film will act similarly to an internal adhesive bandage. A wet, hydrogel film is placed over a tendon after surgical repair and helps reduce any fibrous soft tissue that may develop around it. The team is in the final stages of product development, but there is still much to be done.
As for a name, a long list lines a marker board.
The foundational technology came from the Schmidt Lab of biomedical engineer Christine Schmidt at the University of Texas at Austin in 2008. Biomedical engineer Sarah Mayes, now Alafair’s director of research and development, worked on the gel technology while earning her Ph.D. in the Schmidt Lab. Her role entailed taking the hydrogel technology from “bench to bedside” and getting it from the research lab into a preclinical and clinical setting.
“Dr. Schmidt knew the hydrogel technology was potentially patentable, advancing something already on the market,” said Mayes. Schmidt then initiated a relationship with Dr. Daniel Peterson, a practicing neurosurgeon, who liked the initial film and, according to Mayes, was interested in starting a medical device company.
“We performed a small, successful animal trial and this gave me enough reassurance to form the company and pursue licensing of the technology,” said Peterson who now acts as CEO.
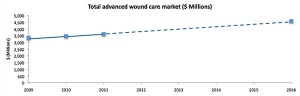
Graphy by Alafair Biosciences, data by BCC Research
The advanced wound care market is growing rapidly, with projections for continued growth. Advanced wound care includes: wound healing devices, equipment, dressings, sealants and anti-adhesion products.
Alafair added a third founder when it launched in February 2011. John Joyoprayitno, an entrepreneur who worked with Peterson on past projects, is now the company’s chief operating officer. Scott Zawko came on as, chief of scientific research, in 2012 and Bhushan Hole joined as product manager in 2013.
Founding Alafair and moving the hydrogel technology out of the university allowed the team to go after the product’s commercial potential. “We licensed the technology so that we could raise money for the commercialization,” said Mayes. “This licensing agreement gives Alafair all rights to use the technology from Dr. Schmidt’s lab.”
Peterson said he has the help of his team to juggle his demanding schedule. “Certainly practicing medicine full-time and being CEO of a startup places a lot of demands on my time. Fortunately, my team at Alafair is extremely special,” said Peterson. “Everyone is committed, responsible, diligent, and they take care of most everything and just tell me where to be and when. I focus time on each specific area according to the demands at that time.”

Alafair Biosciences
Alafair researchers Scott Zawko and Sarah Mayes review current technology literature during a staff meeting. Mayes says Alafair is always interested in new technologies that may expand their product portfolio.
“You can’t start a company like this if you don’t dream big,” said Mayes, who says the team is looking into wound healing both internally and externally. Mayes said Alafair’s hydrogel film advances the technology already on the market, because their product can be applied directly to the tendon wet, setting it apart and making the procedure easier on surgeons.
Their immediate goal is to get their medical hydrogel film through product development and into the market to healthcare professionals. In order to do this, each of the five staff members wear multiple hats and have their phones on and charged at all times.
“The medical device business is a long and slow path to market when compared to software or other technologies,” said Peterson. “However, the rewards can be very satisfying.”
While Peterson, Mayes and Zawko focus on the development of the hydrogel film and additional technologies, including tensile tests and animal necropsies, Peterson, Joyoprayitno, and Hole spend their time on approvals, marketing, and very importantly, locating funding and investors.
The company is a member of the Austin Technology Incubator, an organization associated with the University of Texas at Austin that assists startup businesses with finding funding and other forms of support. Hole said ATI provided Alafair with numerous forms of support, including office space, market research, and assistance raising funds.
Joyoprayitno said Austin is a great place for a company like Alafair to find support. “You have a blossoming community of people that are really supportive of life science ventures, medical device ventures,” he said. “There’s a push in the community to grow the life sciences industry.”
“The other thing that can’t be overlooked is the money,” said Joyoprayitno. “There’s a lot of money and a strong economy in Texas. It’s a great place to start and operate a business. Thanks to the strong economy, technology startups seem to gain more support locally than in other areas where there is more competition and wavering economies. [In Austin] you have a lot of angel investors who are willing and looking to invest in early stage companies, and willing to take on the risk that comes along with them.”
Alafair’s initial product is being developed for use in tendon repair surgery, but additional products derived from this platform technology are under development for various other advanced wound healing applications. A product like this could be extremely beneficial to patients, according to Dr. Mark Larson, a gastroenterologist at the Mayo Clinic in Rochester, Minn
For example, Larson said that a patient might undergo a straightforward operation, such as removal of a gallbladder. Then, years later, because of scar tissue that has formed in the abdomen, develop a narrowing or obstruction of the small intestine, another major operation would be required.
“The problem is real, and quite significant, both in frequency of occurrence, and in severity of impact,” he said.
Alafair is setting a September 2015 product launch date for the hydrogel film technology. Zawko, whose roles involve running the research lab and making samples of the film for testing, says he feels confident that they can make the date, but there is a lot of work to be done in the meantime.
“We are trying to be as aggressive as possible to meet it,” said Zawko. “There are a lot of different activities going on all at once. And if something is either delayed or different than we expected it has to be re-thought.”
Joyoprayitno said his favorite part of working at Alafair isn’t just the initial hydrogel film; it’s creating a platform with the potential to do many things.
“What we’re planning to do, is to create something that we’re going to keep building. It’s fun to watch it grow” he said “It really keeps me motivated, keeping my eye on the big picture.”